Glucose homeostasis involves a complex interplay of hormones, primarily insulin and glucagon, from the pancreas. Insulin facilitates glucose uptake and storage, while glucagon increases blood glucose during fasting. G protein-coupled receptors (GPCRs) play an essential role in this regulation, particularly GLP-1R, which enhances insulin secretion. Additionally, lifestyle factors like physical activity can greatly impact insulin sensitivity and inflammation, vital for maintaining glucose levels. Exploring these mechanisms further reveals deeper insights into metabolic health.
Key Takeaways
- Glucose homeostasis is primarily regulated by the pancreas through insulin and glucagon secretion, maintaining blood glucose levels between 4–10 mM.
- G protein-coupled receptors (GPCRs) play a crucial role in glucose control, particularly GLP-1R, which enhances insulin secretion and suppresses glucagon release.
- Lifestyle factors, such as physical activity and dietary modifications, significantly influence insulin sensitivity and glucose utilization, impacting overall glycemic control.
- Chronic low-grade inflammation disrupts insulin signaling, leading to insulin resistance and impaired glucose homeostasis, often seen in type 2 diabetes mellitus (T2DM).
- Future research focuses on neural mechanisms and innovative therapies, such as GLP-1 receptor agonists, to enhance glucose regulation and manage metabolic disorders.
Overview of Glucose Homeostasis
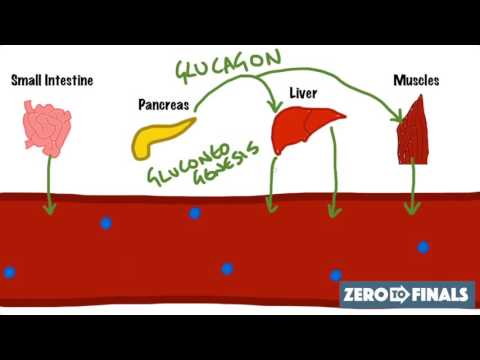
Glucose homeostasis is essential for maintaining your body's energy balance, as it guarantees that blood glucose levels remain within a narrow range of 4–10 mM (70–110 mg/dL). This regulation involves the coordinated actions of various organs, including the pancreas, which houses pancreatic beta cells that secrete insulin. Insulin promotes glucose disposal, glycogen synthesis, and lipogenesis, ensuring that your body effectively utilizes glucose. In contrast, during fasting or stress, counter-regulatory hormones like glucagon elevate blood glucose levels to meet energy demands. Disruptions in this hormonal balance can lead to metabolic syndrome and type 2 diabetes mellitus (DM2), emphasizing the importance of maintaining glucose homeostasis for overall health and metabolic efficiency. Understanding these mechanisms is critical for managing glucose regulation effectively.
Hormonal Regulation of Blood Glucose Levels
The regulation of blood glucose levels hinges on a complex interplay of hormones that respond to the body's metabolic needs. Insulin, secreted by pancreatic beta cells, decreases blood glucose levels by promoting glucose uptake in tissues, stimulating glycogen synthesis, and inhibiting gluconeogenesis. In contrast, glucagon, released by pancreatic alpha cells, increases blood glucose levels during fasting by stimulating glycogenolysis and gluconeogenesis. Somatostatin, produced by delta cells, plays a balancing role in glucose homeostasis by inhibiting both insulin and glucagon secretion. Additionally, incretin hormones like GLP-1 and GIP enhance insulin secretion post-meal and slow gastric emptying. Together, these hormones guarantee precise hormonal regulation of blood glucose levels, maintaining metabolic stability and overall health.
The Role of GPCRs in Glucose Control
While many factors influence glucose homeostasis, G protein-coupled receptors (GPCRs) emerge as critical players in this regulatory process. Among the approximately 30 identified GPCRs, the GLP-1 receptor (GLP-1R) is particularly essential, as it enhances insulin secretion and suppresses glucagon release within pancreatic β-cells, effectively lowering blood glucose levels. Additionally, the GIP receptor (GIPR) also stimulates insulin secretion but may paradoxically elevate glucagon levels in those with type 2 diabetes, illustrating its intricate role in glucose regulation. Free Fatty Acid receptors (FFARs) further modulate incretin hormone release, influencing insulin secretion via various signaling pathways. Targeting GPCRs like GLP-1R presents promising avenues for diabetes treatment, improving insulin secretion and overall glucose control.
Impact of Lifestyle on Glucose Homeostasis
Your lifestyle choices play an essential role in regulating glucose levels, with physical activity enhancing insulin sensitivity and glucose utilization. Conversely, prolonged sedentary behavior greatly elevates the risk of dysglycemia and type 2 diabetes. Additionally, dietary modifications, alongside increased movement, can lead to remarkable improvements in glycemic control, underscoring the importance of an active and balanced lifestyle for ideal glucose homeostasis.
Physical Activity Benefits
Engaging in regular physical activity is essential for enhancing glucose metabolism and maintaining glucose homeostasis, especially considering that many adults fall short of recommended activity levels. Here are three key benefits of physical activity:
- Improves insulin sensitivity – Regular exercise facilitates glucose uptake in muscle tissues, lowering blood glucose levels.
- Reduces cardiometabolic risk – Increased activity helps manage weight, particularly in obese individuals, thereby mitigating risks associated with diabetes.
- Counters sedentary behavior – Breaking prolonged inactivity with short bouts of movement can alleviate insulin resistance and support glucose regulation.
Sedentary Behavior Risks
Physical activity plays an essential role in maintaining glucose homeostasis, but neglecting movement can lead to significant health risks. Sedentary behaviors, characterized by low energy expenditure, are linked to increased dysglycemia and a heightened risk of type 2 diabetes. With about one in three adults meeting recommended activity levels, sedentary lifestyles are prevalent and detrimental to insulin sensitivity and glucose uptake. Regular physical activity enhances glycemic control and combats insulin resistance, critical for metabolic health. Additionally, breaking prolonged sitting with short activity bouts can mitigate these risks. As a result, adopting a whole-of-day movement approach is fundamental for preserving glucose homeostasis and preventing chronic diseases associated with sedentary behaviors. Prioritizing physical activity is essential for maintaining ideal metabolic function.
Dietary Influence on Regulation
While dietary choices markedly impact glucose homeostasis, the interplay between nutrition and lifestyle factors can either promote or hinder metabolic health. Here are three critical aspects to reflect on:
- High intake of processed sugars exacerbates insulin resistance.
- Regular physical activity enhances insulin sensitivity and glucose uptake.
- A balanced diet is essential for maintaining a healthy weight.
Adopting healthier dietary habits can greatly lower the risk of metabolic syndrome and dysglycemia. Research shows that increasing physical activity levels—currently met by only 33% of adults—can effectively improve glucose metabolism. Breaking sedentary behavior with short bursts of activity further supports glucose homeostasis. Consequently, integrating a balanced diet with consistent physical activity is crucial for optimizing metabolic health and preventing type 2 diabetes mellitus (T2DM).
Pathological Changes in Diabetes and Treatment Approaches
In diabetes, dysglycemia arises from complex mechanisms involving disrupted insulin signaling and inflammation, which notably affect glucose homeostasis. You should consider the therapeutic potential of targeting G-protein coupled receptors (GPCRs) to improve insulin sensitivity and manage inflammation-induced insulin resistance. By exploring interventions such as GLP-1R agonists and leukotriene B4 receptor modulation, effective treatment strategies can be developed to address these pathological changes.
Dysglycemia and Its Mechanisms
Dysglycemia, characterized by abnormal blood glucose levels, serves as a crucial indicator of metabolic disruptions, particularly in the context of type 2 diabetes mellitus (T2DM). Key mechanisms contributing to dysglycemia include:
- Insulin resistance leading to impaired glucose uptake in peripheral tissues.
- Elevated glucagon levels promoting hepatic gluconeogenesis and exacerbating hyperglycemia.
- Pathological changes in glucose homeostasis, resulting in impaired glucose tolerance and increased fasting glucose levels.
These disturbances not only complicate glycemic control but also heighten the risk of long-term complications, such as cardiovascular disease and neuropathy. Lifestyle interventions remain essential therapeutic targets, considerably reducing the risk of dysglycemia and improving overall metabolic health. Understanding these mechanisms is crucial for effective diabetes management and prevention strategies.
GPCRs as Therapeutic Targets
Given the intricate role that G protein-coupled receptors (GPCRs) play in regulating glucose homeostasis, they represent promising therapeutic targets in the management of diabetes. Specifically, GLP-1 receptor (GLP-1R) agonists enhance insulin secretion and suppress glucagon release, effectively lowering blood glucose levels. Additionally, targeting receptors such as GPR119 and FFAR4 can bolster the incretin system's activity, addressing insulin resistance. Research also highlights the role of LTB4 receptors in inflammation and insulin resistance, where knockout models show improved glucose tolerance, indicating another potential target. By focusing on these GPCRs, innovative strategies can be developed to restore glucose homeostasis, thereby improving overall diabetes management and reducing complications associated with this chronic condition.
Inflammation and Insulin Resistance
While the interplay between inflammation and insulin resistance is complex, it's clear that chronic inflammation greatly contributes to the pathophysiology of type 2 diabetes mellitus (T2DM). Here are three key points to take into account:
- Pro-inflammatory cytokines disrupt insulin signaling, leading to decreased glucose uptake in muscle and adipose tissues.
- Chronic low-grade inflammation impairs pancreatic β-cell function, exacerbating insulin resistance.
- Lifestyle interventions, like increased physical activity and dietary changes, can reduce inflammation and improve insulin sensitivity.
Targeting G protein-coupled receptors (GPCRs) has shown promise in enhancing insulin sensitivity and mitigating inflammation. Therefore, addressing inflammation through both pharmacological and lifestyle strategies is essential in managing T2DM effectively, ultimately improving glucose homeostasis and patient outcomes.
Neural Mechanisms and Their Influence on Glucose Regulation
The intricate neural mechanisms governing glucose regulation reveal how the brain integrates various sensory inputs to maintain homeostasis. The brain's role in glucose homeostasis is essential, modulating insulin secretion through autonomic neural circuits. Specifically, vagal nerve activation communicates directly with pancreatic islets, stimulating insulin release and influencing peripheral glucose regulation. The ventromedial nucleus of the hypothalamus (VMN) is significant for sensing glucose levels and coordinating counter-regulatory responses during fluctuations. Vagal sensory neurons relay vital information about blood glucose levels to the brain, enabling modulation of autonomic responses that affect hepatic glucose production. Advanced techniques like optogenetics provide insights into specific neural circuits, enhancing our understanding of their roles in glucose regulation and responses to hypoglycemia.
Future Directions in Glucose Homeostasis Research
As research into glucose homeostasis evolves, focusing on unique contexts, such as high altitudes, offers intriguing insights that could reshape our understanding of glucose regulation. Consider these key areas for future exploration:
- The impact of high altitude on insulin secretion and blood glucose levels.
- The role of physical activity in preventing type 2 diabetes through improved glucose tolerance.
- Advancements in GLP-1 receptor agonists as therapeutic strategies for metabolic disorders.
Investigating these aspects could clarify how environmental factors influence glucose homeostasis. Additionally, employing model organisms will enhance our understanding of neural regulation mechanisms. Finally, refining measurement methods for blood glucose levels at high altitudes is essential to avoid artifacts that could skew glycemic assessments, ensuring accurate data for future studies.
Frequently Asked Questions
What Is the Mechanism of Glucose Homeostasis?
Glucose homeostasis relies on insulin secretion and glucagon release to maintain balanced blood sugar levels. When you eat, insulin promotes muscle uptake of glucose and glycogen synthesis in the liver, while nutrient absorption enhances this process. In fasting states, glucagon stimulates liver function to release glucose into the bloodstream. Feedback loops involving these hormones guarantee hormonal balance, optimizing metabolic pathways to regulate glucose levels effectively and maintain energy homeostasis.
What Are the Four Main Steps of Glucose Homeostasis?
Imagine your body as a finely-tuned orchestra. To maintain harmony, four main steps play essential roles: first, insulin secretion prompts glucose uptake in tissues; next, glycolysis transforms glucose into energy; then, gluconeogenesis in liver function creates glucose from non-carb sources; finally, glycogen synthesis and degradation regulate storage and release. Together, these metabolic pathways guarantee energy balance through hormonal regulation and feedback loops, influenced by dietary choices and exercise, keeping blood sugar stable.
What Are the Mechanisms Involved in Blood Glucose Homeostasis During Exercise?
During exercise, your body regulates blood glucose through various mechanisms. Hormonal regulation, including increased glucagon and epinephrine, drives glycogen breakdown and enhances glucose availability. Muscle contraction boosts insulin sensitivity, promoting glucose uptake by active tissues. Energy expenditure increases carbohydrate utilization, while endurance training fosters metabolic adaptation, optimizing glucose handling. Together, these processes guarantee stable blood glucose levels, facilitating sustained physical performance and preventing hypoglycemia during prolonged activity.
How Does Glucose Metabolism Demonstrate a Homeostatic Mechanism?
It's like a well-orchestrated dance, where glucose metabolism keeps your body in sync. Insulin secretion triggers glucose uptake, ensuring your cells have energy. The liver functions as a key player, balancing blood sugar through feedback loops and hormonal regulation. When all's well, metabolic pathways harmonize, supporting energy balance. However, a homeostatic imbalance can disrupt this rhythm, leading to issues like insulin resistance, which hampers cellular metabolism and affects overall health.